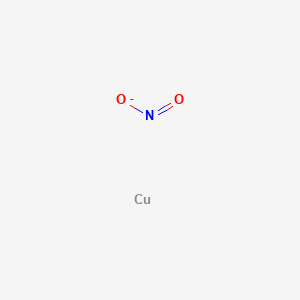
Also note that this combination of nitrogen and oxygen has no electric charge specified so it is not the nitrite ion. Aluminum hydroxide AlOH3 33.

Pin By Pradeep Wickramanayake On Science Chemistry Positivity Math
Chemical formula for water is H₂O which means that water is a compound which is formed by the combination of 2 proportions of H and 1 proportion of O.

What is the chemical formula for copper i nitrite. The use of SO2 to reduce Cr6 to Cr3 in an acidic solution. The reward for building a correct chemical compound is receiving a number of points based on the number value of the cards. Formula writing rules to write the correct chemical formulas for each compound.
Sodium thiosulfate ____Na2S2O3_____ 3. FeBrBr the algebraic sum of the oxidation numbers is 2 2 x -1 0. Sodium nitrite is an inorganic compound with the chemical formula NaNO 2It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopicFrom an industrial perspective it is the most important nitrite salt.
Iron III oxide Fe2O3 32. For example if you see the formula BaNO 3 2 you may recognize the NO 3 part as the nitrate ion NO 3. 68 CuOH copper I hydroxide 69 ZnNO 2 2 zinc nitrite 70 V 2 S 3 vanadium III sulfide Write the formulas for the following chemical compounds.
CopperII sulfate also known as copper sulphate are the inorganic compounds with the chemical formula Cu SO 4 H 2 O x where x can range from 0 to 5The pentahydrate x 5 is the most common form. The first box is the intersection between the zinc cation and the chloride anion so. WORKSHEET- FORMULAS NAME _____ WRITE THE CHEMICAL FORMULA FOR EACH OF THE FOLLOWING.
For fires involving potassium nitrate extinguish with dry chemical CO2 Halon water spray or fog. List of 131 compounds in the Solution Calculator database for which the density function is defined are for the Perrys Chemical Engineers Handbook. Ionic or Covalent Chemical Formula 1 copper II chlorite 2 sodium hydroxide 3 nitrogen dioxide 4 cobalt III oxalate 5 ammonium sulfide 6 aluminum cyanide 7 carbon disulfide.
If water is used apply from as far a distance as possible. Give the name for each compound given its chemical formula. In order to simplify the formula a subscript is used to indicate the number of bromine atoms required.
1 Barium sulfide 1 _____ 26 Aluminum bisulfide 26 _____. Also potassium nitrate presents an explosion risk when shocked or heated and may produce poisonous gases in a fire. EPA sets legal limits on over 90 contaminants in drinking water.
Chemical formulae provide a way to represent any chemical substance using the symbol of the elements present in it. Chemical Reaction Formula Atomic Mass Formula Chemical Formula Enthalpy Formula Entropy Formula Molality. Compound Name Type of Compound.
Use the stock form for the transition metals. Write the chemical formula for each of the following compounds. Sodium nitrite NaNO2 31.
The chemical formula of a compound is a symbolic representation of its chemical composition. In addition to physically building the chemical compound with the cards the player has obtained he or she must also write the correct chemical formula and the correct name of the chemical compound. Chemical Formula Writing Worksheet Solutions Write chemical formulas for the compounds in each box.
Sulfur dioxide ____SO2_____ 2. Armed with a basic Periodic table students can begin to write chemical formulas in one easy lesson. An example of a formula that includes water of hydrate is that of copperII sulfate pentahydrate.
This formula as written is in an inconvenient form since the formula of bromide appears twice. Older names for this compound include blue vitriol bluestone vitriol of copper and Roman vitriol. If fire becomes uncontrollable consider evacuation of one-half.
This is common when dealing with any transition metal or rare earth. Ammonium hydroxide NH4OH 34. Chemical formulae provide insight into the elements that constitute the molecules of a compound and also the ratio in which the atoms of these elements combine to form such molecules.
Thus for the formula. The formula is written as CuSO 4 5H 2 O. Silver sulfide Ag2S 23.
The pentahydrate CuSO 4 5H 2 O the most commonly encountered salt is bright blue. Give the formula for the following. Complete these in lab and on your own time for practice.
Nickel II carbonate NiCO3 24. CobaltII Nitrite CO2 Carbon Dioxide CoCl2 CobaltII Chloride COCl2 Phosgene CoSO4 CobaltII Sulfate CrOH3 Chromium Hydroxide Cr2O3 ChromiumIII Oxide CrCl3 ChromiumIII Chloride CS2 Carbon Disulfide CsCl Caesium Chloride CuCN2 CopperII Cyanide CuNO32 CopperII Nitrate CuOH2 CopperII Hydroxide Cu2O CopperI Oxide Cu2S Copper. Potassium carbonate K2CO3 22.
11 silicon dioxide SiO 2 12 nickel III sulfide Ni 2 S 3 13 manganese II phosphate Mn 3 PO 4 2 14 silver acetate AgC 2 H 3 O 2 15 diboron tetrabromide B 2 Br 4 16 magnesium sulfate. This is the quickest and easiest way to learn. The legal limit for a contaminant reflects the level that protects human health and that water systems can achieve using the best available technology.
Provided below is a list of the chemical formulas of some common chemical compounds along with their molecular weights. The names are found by finding the intersection between the cations and anions. Zinc Nitrate Formula Copper II Sulfate Formula Aluminum Sulfide Formula Sulfur Trioxide Formula Sodium Bromide Formula Copper II Oxide Formula Aluminum Nitrate Formula Nitrite Formula Magnesium Nitride Formula Barium.
71 silicon dioxide SiO 2 72 nickel III sulfide Ni 2 S 3 73 manganese II phosphate Mn 3 PO 4 2 74 silver acetate AgC 2. A chemical formula is an expression that represents the element in that compound along with its relative proportion in the compound. Name of Compound individual ions Formula lithium cyanide Li CN LiCN iron III sulfate Fe3 SO 4 2 Fe 2SO 4 3 calcium iodide Ca2 I CaI 2 tin IV dichromate Sn4 Cr 2O 7 2 SnCr 2O 7 2 silver nitrite Ag NO 2 AgNO 2 copper II acetate Cu2 C 2H 3O 2 CuC 2H 3O.
Reduction treatment the opposite of oxidation treatment wherein a reductant is used to lower the valence state of a pollutant to a less toxic form. Learn how to write the chemical formula of a variety of chemical compounds using the arms and link method. Chemical reaction in which an atom or molecule gains an electron.
Perry Perrys Chemical Engineers Handbook McGraw-Hill 2 99-118 2008. 8 CuOH copperI hydroxide 9 ZnNO 2 2 zinc nitrite 10 V 2 S 3 vanadiumIII sulfide Write the formulas for the following chemical compounds. You should complete this by Sunday.
Decrease in positive valence. Nomenclature a collection of rules for naming things is important in science and in many other situationsThis module describes an approach that is used to name simple ionic and molecular compounds such as NaCl CaCO 3 and N 2 O 4The simplest of these are binary compounds those containing only two elements but we will also consider how to name ionic compounds containing. In this example two bromine atoms.
Chemical Formula Nomenclature Practice. Second if you recognize the formula of a polyatomic ion in a compound the compound is ionic. Addition of hydrogen to a molecule.
Note that the name of the salt includes the oxidation state of copper.

How To Write The Formula For Copper Ii Nitride Youtube

Chemical Formula Science Notes Chemistry Lessons Teaching Chemistry

How To Write The Formula For Copper I Nitride Youtube

How To Write The Formula For Copper I Nitrate Youtube

Write The Chemical Formula Of Copper Nitrate Youtube

Solved 16 What Is The Correct Chemical Formula For Copper Chegg Com

How To Write The Formula For Copper Ii Nitrate Youtube